JAMB Exam Questions for day 2, If you are searching for today’s Jamb exam question for Jamb day 2 candidate which was written today then kindly check out these questions that were released here.
JAMB 2022 Exam Questions for day 2 and Answers For Jamb day 2 are what is available here. Candidates who took the Jamb exam day 2 as of when Jamb began provided some questions they come across during the Jamb exam day 2 all the questions to JAMB Exam Day 2 on Mathematics, Biology, Physics, and Chemistry questions for 2022 Jamb Exam Day 1 are released here and you check them here in this article.
JAMB exam questions for Jamb day 3 will soon be released here and you need to know what questions to expect and the solutions to 2022 JAMB UTME, Economics, Biology, Physics, e.t.c. then you are on the right page.
We have provided both the JAMB Exam day 1 and Jamb exam day 2 Questions for Maths, Physics, English, Economics, Civics, Biology e.t.c and what you should know about the questions that will appear in the actual JAMB Day 1, JAMB Day 2, JAMB Day 3, JAMB Day 4, JAMB Day 5 for 2022/2023 examination which will commence soon.
JAMB 2022 UTME Questions and Answers can be found right on this page. All the JAMB Exams Questions for all the days are right on this website and you can access them easily as you continue to read this article.
Jamb Mathematics Questions For Exam Day 2 Morning, Afternoon, and Evening
Solve the following equations
4x – 3 = 3x + y = 2y + 5x – 12
A. 4x=5, y= 2
B. x=2, y=5
C. x=-2, y=-5
D. x=5,y=-2 E. x=-5,y=-2
If x = 1 is the root of the equation
x3 – 2×2 – 5x + 6, find the other roots
A. -3and2
B. –2 and2
C. 3and–2
D. 1and 3
E. –3and 1
If x is jointly proportional to the cube of y and the
fourth power of z. In what ratio is x increased or
decreased when y is halved and z is doubled?
A. 4:1increase
B. 2:1increase
C. 1:4 decrease
D. 1: 1 nochange
E. 3: 4decrease
Find the angle of the sectorsrepresenting each item in
a pie chart of the following data. 6,10,14,16,26
A. 150,250,350,400,650,
B.600,1000,1400,1600,2600
C. 60,100,140,160,260,
D.300,500,700,800,1300
E. None ofthe above
The scores of 16 students in a Mathematics test are
65,65,55,60,60,65,60,70,75,70,65,70,60,65,65,70
What is the sum of the median and modal scores?
A. 125
B. 130
C. 140
D. 150
E. 137.5
The letters of the word MATRICULATION are cut and
put into a box. One of the letters is drawn at random from
the box. Find the probability of drawing a vowel.
A. 2/13
B. 5/13
C. 6/13
D. 8/13
E. 4/13
Correct each of the numbers 59.81789 and 0.0746829 to
three significant figures and multiply them, giving your
answer to three significant figures.
A. 4.46
B. 4.48
C. 4.47
D. 4.49
E. 4.50
If a rod of length 250cm is measured as 255cm longer in
error, what is the percentage error in measurement?
A. 55
B. 10
C. 5
D. 4
E. 2
If(2/3)m (3/4)n = 256/729, find thevalues ofm and n
A. m=4,n= 2
B. m=-4,n=-2
C. m=-4,n= 2
D. m=4,n=-2
E. m=-2,n= 4
Without using tables find the numerical value of log7
49+ log7(1/7)
A. 1
B. 2
C. 3
D. 7
E. 0
Factorize completely 81a4 – 16b4
A. (3a + 2b) (2a – 3b) (9a2 + 4b2)
B. (3a – 2b) (2a – 3b) (4a2- 9b2)
C. (3a – 2b) (3a – 2b) (9a2 + 4b2)
D. (3a – 2b) (2a – 3b) (9a2 + 4b2)
E. (3a – 2b) (2a – 3b) (9a2- 4b2)
One interior angle of a convex hexagon is 1700
and each of the remaining interior angles is equal to x0
. find x
A. 1200
B. 1100
C. 1050
D. 1020
E. 1000
PQRS is a cyclic quadrilateral in which PQ = PS. PT is a
tangent to the circle and PQ makes an angle of 500 with
the tangent as shown in the figure below. What is the
size of QRS?
A. 500
B. 400
C. 1100
D. 800
E. 1000
A ship H leaves a port P and sails 30km due South.
Then it sails 60km due west. What is the bearing of H
from P?
A. 26034’
B. 243026’
C. 116034’
D. 63026’
E. 2400
In a sample survey of a university community the
the following table shows the percentage distribution of
the number of members per household.
A. 4
B. 3
C. 5
D. 4.5
E. None
On a square paper of length, 2.524375cm is inscribed a
square diagram of length 0.524375. find the area of the
paper not covered by the diagram correct to 3 significant
figures.
A. 6.00cm2
B. 6.10cm2
C. 6.cm2
D. 6.09cm2
E. 4.00cm2
If f(X) = 1 + x – 1 find f(1-x)x-1 x2-1
A. 1/x + 1/(x+2)
B. x +1/(2x -1)
C. -1/x – 1/(x-2)
D. -1/x + 1/(x2-1)
Jamb Biology Questions For Exam Day 2 Morning, Afternoon, and Evening
The male toad differs from the female by having
A. vocal sacs.
B. shorter hind limbs.
C. longer forelimbs.
D. bulging eyes.
E. nictating membrane.
Mosses, liverworts, and ferns can be grouped together because they
A. are all aquatic plants.
B. all grow in deserts.
C. are seedless plants.
D. have undifferentiated plant bodies.
E. all produce colorless flowers.
Spirogyra and Mucor can be grouped together as Thallophyta because.
A. they are unicellular organism
B. their spores could be dispersed by wind
C. they are capable of living independent lives
D. they reproduce sexually only
E. their bodies are made up of thallus and filaments alternatively.
Which of the following invertebrates does NOT
possess antennae?
A. Centipede
B. Crustacean
C.Millipede
D. Insect
E. Spider
Which of the following is INCORRECT? The prothallus of a fern
A. is a flattened heart-shaped structure.
B. is green because its cells contain chloroplasts
C. is the dominant plant
D. bears the sexual organs
E. is attached to the ground by numerous rhizoids.
Which of the following cell constituents is NOT common in both plants and animals?
A. Mitochondria
B. Chloroplasts
C. Ribosomes
D. Golgi apparatus
E. Vacoules.
The character-producing factors in living organisms are
A. chromomeres
B. alleles
C. chromatids
D. chromosomes
E. genes.
A mixture of mercurous and mercuric nitrates is added to a food substance. A white precipitate is formed which on gentle heating turns red. The food substance is
A. protein
B. oil
C. Carbohydrate
D. Fat
E.Fatty acid.
The mammalian organ through which nourishment and oxygen diffuse into a developing embryo is called
A. amnion
B. chorion
C. umbilical cord
D. oviduct
E. placenta
Which of the food chains are the correct sequences?
A. weeds, tadpoles, beetle, man
B. weeds, tadpoles, fish, beetle, man
C. tadpoles, beetles, weeds, man, fish
D. man, fish, beetles, weeds
33. The primary and secondary hosts respectively of bilharzia are
A. fish and man
B. man and dog
C. snail and man
D. man and snail
The origin of mineral particles in the seed is
A. humus
B. water
C. micro-organism
D. organic matter
The initial volume of water in the bag of dry soil was 50ml and the amount that drained through was 35ml, the percentage water content of the fully soaked soil is therefore
A. 46.7
B. 25.0
C. 20.0
D. 30.0
From the following list of types of mutation, identify the one that is not hereditary
A. genetic mutation
B. somatic mutation
C. germinal mutation
D. gametic mutation
In a cell, digestive enzymes mostly occur in
A. ribosomes
B. lysosomes
C. mitochondria
D. plastids
Jamb Physics Questions For Exam Day 2 Morning, Afternoon, and Evening
In storing magnets, keepers are used to
A) reduced self-demagnetization
B) cancel the effect of the earth’s magnetic field
C) protect the magnet from the stray electric field
D) increase the strength of the magnets
What determines the polarity at the ends of an electromagnet? The
A) the magnitude of the current passing through the wire
B) material of the core of the magnet
C) material of the coil
D) direction of current in the wire
A) 1B) 2
C) 3
D) 5
The phenomenon by which two light atomic nuclear combine to form a heavy nuclide with the release of energy is known as
A) radioactivity
B) nuclear fusion
C) nuclear fission
D) chain reaction
In a p-type semiconductor, the
A) number of holes is equal to the number of electrons
B) electrical resistivity increases
C) electrons are the majority charge carriers
D) holes are the majority charge carriers
Let Δx be the uncertainty in the measurements of position and Δp the uncertainty in the measurement of momentum. The uncertainty principle relation is given as
A) Δx . Δp = h
B) Δx . Δp ≤ h
C) Δx . Δp ≥h
D) Δx . Δp > h
Two objects of masses 80kg and 50kg are separated by a distance of 0.2m. If the gravitational constant is 6.6 x 10-11Nm kg, calculate the gravitational attraction between them.
A) 4.9 × 10-9N
B) 1.3 × 10-6N
C) 6.6 × 10-8N
D) 6.6 × 10-6N
E) 2.6 × 10-9N
1kg of boiling water at 100°C is poured into an aluminium hot water bottle at 16°C. The temperature of the water quickly falls to 96°C. Assuming that there is no loss of heat, the mass of the bottle is
A) 0.25kg
B) 0.5kg
C) 0.05kg
D) 0.75kg.
The resultant of two forces is 50N. If the forces are perpendicular to each other and one of them makes an angle of 30° with the resultant, find its magnitude
A) 57.7N
B) 25.0N
C) 100.0N
D) 57.7N
E) 43.3N
Two capacitors C1 and C2 are connected as shown in the diagram. The capacitance C2 is twice C1 when the key is opened the energy stored up in C1 is W. If the key is later closed and the system is allowed to attain electrical equilibrium, the total energy stored in the system will be
A) 12W
B) 23W
C) W
D) 2W
E) 3W
Which of the following is stored by dry Leclanche cell?
A) chemical energy
B) nuclear energy
C) solar energy
D) heat energy
E) electrical energy
When a brick is taken from the earth’s surface to the moon, its mass
The pair of physical quantities that are scalar only are
A) Volume and area
B) Impulse and time
C) Moment and momentum
D) Length and displacement
A simple pendulum of length 0.4m has a period of 2s. What is the period of a similar pendulum of length 0.8m at the same place?
A) 2√2s
B) √2s
C) 8s
D) 4s
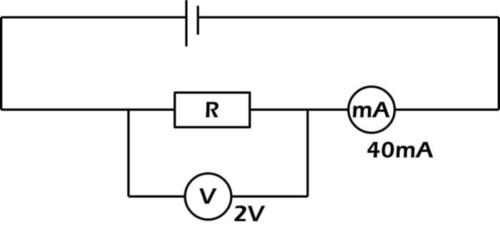
Using the data in the circuit illustrated above, calculate the value of R
A) 0.02 Ω
B) 0.05 Ω
C) 5.00 Ω
D) 20.00 Ω
E) 50.00 Ω
A 90W immersion heater is used to supply energy for 5 minutes. The energy supplied is used to completely melt 160g of a solid at its melting point. Calculate the specific latent heat of the solid.
A) 2.81 Jg-1
B) 6.25 Jg-1
C) 8.89 Jg-1
D) 168.75 Jg-1
E) 5333.33 Jg-1
Jamb Chemistry Questions For Exam Day 2 Morning, Afternoon, and Evening
The minimum amount of energy required for effective collisions between reacting particles is known as
A) Activation energy
B) Bond energy
C) Kinetic energy
D) Potential energy
The bond formed between H2O and H+ to form the hydroxonium H3O+ is
A) Dative
B) Covalent
C) Electrovalent
D) Ionic
An element X forms the following oxides X2O,XO and XO2. This phenomenon illustrates the law of ________.
A) Conservation of mass
B) Definite proportion
C) Mass action
D) Multiple proportion
How many moles of oxygen would contain 1.204×1024 molecules?
NB: Avogadro’s constant (NA) =6.02×1023
A) 1
B) 2
C) 3
D) 4
Which of the following statements about solids is correct?
A) Solid particles are less orderly than those of a liquid
B) Solid have lower densities than liquids
C) Solid particles have greater kinetic energies than those of liquids
D) Solid particles cannot be easily compressed
Which of the following apparatus can be used to measure a specific volume of a liquid accurately?
A) Beaker
B) Conical flask
C) Measuring cylinder
D) Pipette
The general gas equation PVT=K is a combination of
A) Boyle’s and Charles’ laws
B) Boyle’s and Graham’s laws
C) Charles’ and Graham’s laws
D) Dalton’s and Graham’s laws
The spreading of the scent of a flower in a garden is an example of ________.
A) Brownian motion
B) Diffusion
C) Osmosis
D) Tynadal effect
Propane and carbon (IV) oxide diffuse at the same rate because (H = 1.00, C = 12.0, O = 16.0)
A) They are both gases
B) Their molecules contain carbon
C) They have the same relative molecular mass
D) Both are denser than air
The energy which accompanies the addition of an electron to an isolated gaseous atom is
A) Atomization
B) Electronegativity
C) Electron affinity
D) Ionization
An aqueous solution of (NH4)2 SO4 is
A) Acidic
B) Alkaline
C) Amphoteric
D) Neutral
When NH4Cl(s) is dissolved in water, the container feels cold to touch. This implies
A) The process is endothermic
B) The process is exothermic
C) NH4Cl forms a saturated solution
D) NH4Cl is highly soluble in water
The pH of four solutions M, N, Q and R are 2, 6, 8 and 11 respectively.
Which of the following deductions about the solutions is correct
A) The pH of N is increased when the solution is diluted
B) The pH of Q is increased when the solution is evaporated
C) M is the most alkaline solution
D) R is the most acidic solution
Consider the following reaction equation
H3O+(aq) + OH(aq) → 2H2O(l)
The reaction represents
A) Esterification
B) Hydrolysis
C) Neutralization
D) Redox
The refreshing characteristic taste of fizzy drinks is due to the presence of
A) Carbon (IV) oxide
B) Glucose
C) Hydrogen
D) Sodium Citrate
Chemical equilibrium is attained when
A) All the reactants have been completely used up
B) The reaction goes to completion
C) The concentration of reactants and products remain constant
D) The concentration of reactants and products are equal
Which of the following factors will affect the rate of the reaction represented by the following equation?
2HCl(aq) + CaCO3(s) → CaCl2(aq) + H2O(l) + CO2(g)
I. Pressure
II. Concentration
III. Nature of reactants
IV. Temperature
A) I and II only
B) II, III and IV only
C) I, II and III only
D) I, II, III and IV
On evaporation to dryness, 350cm3 of a saturated solution of salt Z gave 55.5g of salt. What is the solubility of the salt? [Z = 101]
A) 1.57 moldm-3
B) 3.14 moldm-3
C) 6.28 moldm-3
D) 12.56 moldm-3
Which of the following salts when dissolved in water will form a solution that will change blue litmus to red?
A) CH3COONa
B) NH4Cl
C) NaCl
D) KCl
Which of the following bonds are broken when ethanol is boiled?
I. Covalent bonds
II. Ionic bonds
III. Hydrogen bonds
A) I only
B) II only
C) III only
D) I, II, III
A compound with the molecular formula CH2O2 is
A) A Carbohydrate
B) A Carboxylic acid
C) An alkanol
D) An ester
The quantity of electricity required to discharge 1 mole of univalent ion is
A) 9600 C
B) 48250 C
C) 96500 C
D) 193000 C
Chlorine water is used as a bleaching agent because it is
A) An acidic solution
B) An alkaline solution
C) An oxidizing agent
D) A reducing agent
Which of the following substances is a non-electrolyte?
A) H2SO4
B) CH3COOH
C) C6H12O6
D) NH4Cl
The oxidation number of sulphur is +4 in
A) Na2S2O3
B) H2SO3
C) H2SO4
D) SO3
Consider the following ionic equation:
Cr2O72- + 14H+ + ne– → 2Cr3+ + 7H2O.
The value of n in the equation is _______?
A) 7
B) 6
C) 3
D) 2
Consider the following half-cell reactions.
Al(s) → Al3+(aq) + 3e–
Cu2+(aq) + 2e– → Cu(s)
The overall equation for the reaction is
A) Al(s) + Cu2+(aq) → Al3+(aq) + Cu(s)
B) 2Al(s) + Cu2+(aq) → 2Al(aq) + Cu(s)
C) 2Al(s) + 3Cu2+(aq) → 3Cu(s) + 2Al3+(aq)
D) 3Al(s) + 2Cu2+(aq) → Cu(s) + 3Al3+(aq)
Amino acids are obtained from proteins by
A) Hydrolysis
B) Oxidation
C) Polymerization
D) Reduction
When compound X is heated with concentrated tetraoxosulphate (VI) acid, it produces an alkene. X is an
A) Alkane
B) Alkanol
C) Alkanoate
D) Alkyne
Ripening of fruits is hastened by using
A) Ethanol
B) Ethane
C) Ethene
D) Ethyne
To get the JAMB DAY 3, JAMB DAY 4, JAMB DAY 5, JAMB DAY 6 2022 JAMB Exam Questions and Answers kindly Join Nkedugists Forum. You can get the answers or work on it.
Note that:
The JAMB Exam Day 1 questions which are posted here are questions you are likely to see in your Jamb day 2, Jamb day 3 or Jamb day 4 it is not a must you will see all but simply work on the Jamb exam day 1 questions it can save you a lot and put a smile on your face.
If you are a candidate who wrote his/her Jamb exam on Jamb day 2, Jamb day 3, or Jamb day 4 e.t.c and you are sure of what questions you saw during your own Jamb exam day and you want to help we are going to drop a link to NKEDUGISTS FORUM WHERE YOU CAN POST THE JAMB EXAM QUESTIONS IN OTHER TO HELP OTHER CANDIDATES.
If actually, this information is awesome and useful to you please kindly share using via Facebook, WhatsApp, Twitter, and Google+




10 Comments
Please am writing my exams on Saturday day 2 please those questions I saw about day 2 are the really going to come out
kindly study it and give us feedback on it when you return from your Jamb center.
Please sir I’m writing my exam on Monday 12:00 can I get question and answer for use of English, Lit_in eng, government and crs please help me
Dz is my whatsapp num
09044303480
I really want to gain an admission this year🙏 may God help and assist you too
I’m tired of staying home about 3years now
I’m done learning work
For sure you are going to gain admission, Follow the questions drop in that article work on them, and get the answer… You will score above 180
Pls am writing my exam on the 10 which of the day question can I go through very well
Pls sir, my WhatsApp
No. 09077188088
Noted
Thanks Soo much I really appreciate u fr all what u do sir
Pls sir I need financial account day 1 nd 2
economics day 2
Noted
Sir am write a mop jamb exam on the 6 of August, sir I don’t know where to start from reading, can I get possible questions on English, maths, physics and chemistry