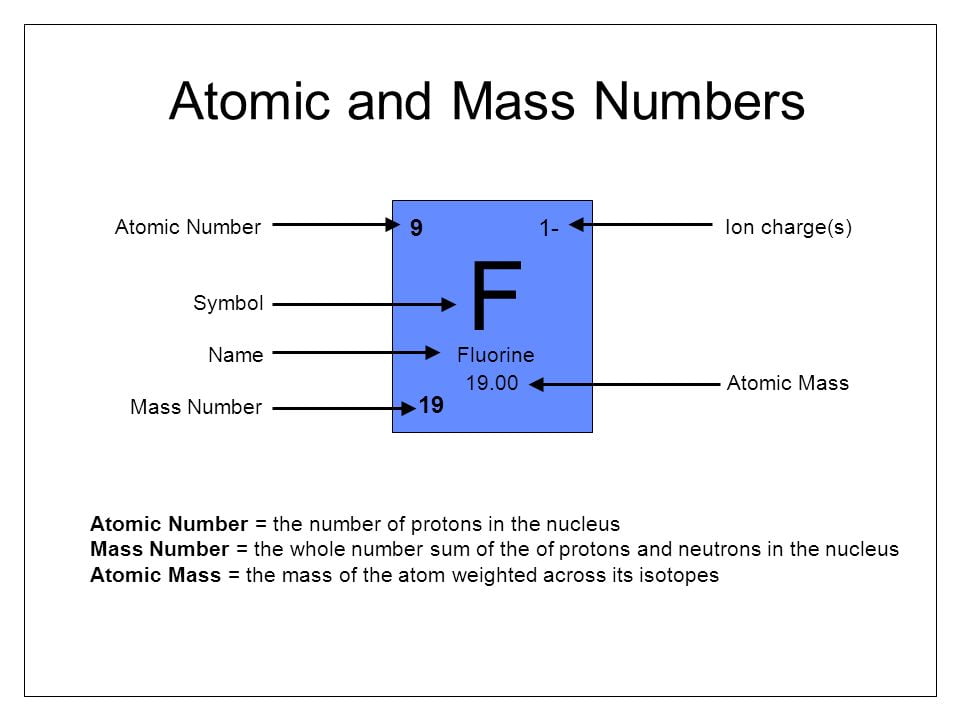
The solution to relative atomic mass: If the atomic number of an element is given you know what the proton is; the same as the atomic number. if that element is neutral you also know that the number of electrons is the same. but if you are only given the atomic number is it possible to find out what the atomic mass is?
In this post, we are providing solutions to some questions on chemistry on particulates Nature Of Matter.
If you are using an essential textbook go to page 65 of your essential textbook you will see the questions there. We solve some of the questions that will think most students have issues with.
Chemistry Problems Questions On Isotopes Relative Atomic Mass
1. How many electrons are in the ion Mass number 19 and atomic number 9
(a)8 (b)9 (c)10 (d)19
2. What is the mass number of an element if its atom contains 10 protons, 10 electrons, and 12 neutrons?
(a)32 (b) 22 (c) 20 (d)10
3. 14.8g of salt dissolved in 250 cm³ of distilled water gives a concentration of 0.80 mol dm-³. Calculate the molar mass of salt.
(a)13.5g mol-¹ (b) 18.5g mol-¹(c) 47.4gmol-¹ (d) 74.0gmol-¹.
4. How many atoms are there in one mole of hydrogen gas? {Avogadro’s constant (NA)=6.02×10²³ mol-¹}
(a) 1.2×10²³ (b) 6.0×10²³ (c) 1.2×10²⁴ (d) 6.0×10²⁴.
6. Neutral atoms of neon with atomic number 10 have the same number of electrons as
(a) 0² (b)Ca²+ (c) K+ (d) Mg²+ .
10. The electronic configuration of an atom is 2,8,8,2, The atomic number of an element is therefore
(a) 10 (b) Z15 (c) 18 (d)20.
(11) Which of the following ions have the same number of electrons as neon?[⁹F,¹⁰Ne, ¹¹Na, ¹²Mg, ¹³AI] I. Na+II.Mg².III. AI³+ IV. F
(a) I and II only (b) II, III and IV only (c) I, II and III and only (d) I, II, III, and IV.
19. What is the mass of 6.02×10²⁴ atoms of magnesium? [Mg=24,Avogadro’s constant =6.02×10²³ atoms mol‐¹]
(a) 240g (b)24g (c)2.4g (d) 0.24g
20. An element X with relative atomic mass 16.2 contains two isotopes ¹⁶8 X a relative abundance of 90% and m X with the relative abundance of 10%.The value of m is (a) 14 (b) 12 (c) 18 (d)16
Answers to Some Questions on Isotopes
How many electrons are in the ion Mass number 19 and atomic number 9
(a)8 (b)9 (c)10 (d)19 The answer is (b)

2. What is the mass number of an element if it’s atom contains 10 protons, 10 electrons and 12 neutrons?
(a)32 (b) 22 (c) 20 (d)10 The answer is (b)

3. 14.8g of salt dissolved in 250 cm³ of distilled water gives a concentration of 0.80 mol dm-³. Calculate the molar mass of salt.
(a)13.5g mol-¹ (b) 18.5g mol-¹(c) 47.4gmol-¹ (d) 74.0gmol-¹.
The answer is (c)

6. Neutral atoms of neon with atomic number 10 have the same number of electrons as
(a) 0² (b)Ca²+ (c) K+ (d) Mg²+ . The answer is (a)

10. The electronic configuration of an atom is 2,8,8,2, The atomic number of elements is therefore
(a) 10 (b) Z15 (c) 18 (d)20. The answer is (d)

(11) Which of the following ions have the same number of electrons as neon?[⁹F,¹⁰Ne, ¹¹Na, ¹²Mg, ¹³AI] I. Na+II.Mg².III. AI³+ IV. F
(a) I and II only (b) II, III and IV only (c) I, II and III and only (d) I, II, III, and IV. The answer is (b)

19. What is the mass of 6.02×10²⁴ atoms of magnesium? [Mg=24,Avogadro’s constant =6.02×10²³ atoms mol‐¹]
(a) 240g (b)24g (c)2.4g (d) 0.24g the answer (a)

20. An element X with relative atomic mass 16.2 contains two isotopes ¹⁶8 X with the relative abundance of 90% and m X with the relative abundance of 10%.The value of m is (a) 14 (b) 12 (c) 18 (d)16
. The answer is (C)

Related Articles: Definition of Isotopes and Relative Atomic Mass (Solutions)