Waec Chemistry Questions and Answers for free to all Weac candidates In Ghana, Liberia, Nigeria, Sierra Leone, and The Gambia. On this page, all the Waec Chemistry questions and answers for 2023 and the most common questions and answers are released here.
Waec Candidates that applied for the West African Examination Council (WAEC) SSCE Examination will write their Waec Chemistry For Science students. All details you need for you to be successful and pass this 2023 Waec Exam will also be given and make sure you read all through.
2023 Waec Chemistry Exam Papers
2023 Waec Chemistry Exam Papers Are
- Waec Chemistry Essay Questions
- Waec Chemistry Objectives Questions,
You are writing the 2 papers in only one day. In this post, the previous Year’s Waec questions and answers for Chemistry are released and the 2023 Waec Chemistry Exam Questions will also be released for those participating in the 2023 Waec examination.
2023 Waec Chemistry Questions and Answers Objective (paper 1)
The 2023 Waec Chemistry questions and answers loading! 2023 Chemistry objective answers Loading!! 2023 Waec Chemistry Theory Answers Loading!!! Kindly bookmark the website for the answers that will be released. or better still reload the site to check if the answers for the 2023 Waec Chemistry questions and answers have dropped.
Previous Year WAEC Chemistry OBJ ANSWERS
CHEM-OBJ
1caacabcdbc
11babaadbdca
21bccaabcdcb
31abcbcccbbc
41abdbbbaabb
Chem-Theory-Answers
==================================
2023 Waec Chemistry Questions and Answers THEORY (paper 2)
The 2023 Waec Chemistry Theory questions and answers are loading! 2023 Chemistry Essay answers Loading!! 2023 Waec Chemistry Theory Answers Loading!!! Kindly bookmark the website for the answers that will be released. or better still reload the site to check if the answers for the 2023 Waec Chemistry questions and answers have dropped.
3d)
VIEW IMAGE HERE – CLICK
3ai)
structural isomerism is the existence of two or more compounds
( known as isomers) with the same molecular formula but
different molecular structures
3ai)
i) tertiary alkanol
ii) secondary alkanol
iii) primary alkanol
3bi)
i) 2[CH3 CH2 COOH ] 2K(s)—->
ii) [CH3 CH2 COO ] K + H2
i) 2[CH3 CH2 COOH ] +2 K(s) ——> 2[ CH3 CH2 COO ] K + H2
ii) CH3 CH2 COOH + C4 H9OH HEAT—->CH3 CH2 COO C4 H9 + H20
H2SO4
iii)CH3 CH2 CH2 CH20H + H^+ KMnO4——> CH3 CH2 CH2 CH2 – 0H EXCESS
3bii)
i) potassium ethanoate,hydrogen
ii) butyl propanoate and water
iii) enthanal
3c)
The percentage composition of the hydrocarbon
= 100
H + C = 100
14.3 + C =100
C= 100 – 14.3
C= 85.7%
================
1a)
nucleons is the collective name for two important sub-atomic
particles:neutrons and protons
1b)
Graham’s law of diffusion states that at a constant temperature
and presure,the rate of diffusion of a gas is inversely proportional
to the square root of it density
1c)
because when aluminum is exposed to moist air,a thin
continuous coating of aluminum oxide is formed, which
prevents futher attack of the aluminium by atmospheric oxygen
and water or steam under normal conditions
1d)
i) electron – affinity
ii) electron – negativity
iii) ionization – energy
1e)
i) the molecule of real gases occupies space and there are forces
of the attraction between them
ii) real gases do liquefy when their temperature droops.
1f)
i)used in seperation of different component of crude oil.
ii)it is used for separating acetonic from water
iii) it is used for obtaining different gases from air for industrial
use.
1g)
i)concentration of ions in electrolyte
ii)the nature of the electrode
iii)the position of ions in the electrochemical series
1h)
i)substitution reaction
ii)addition reaction
1i)
i)carbon dioxide
ii)water vapour
iii) carbon monoxide
1j)
i)centrifugation
ii)sieving
iii)evaporation to dryness
==================================
2a)
i) isotopy
ii) they have the same number of proton (atomic number) but
different mass number
iii) oxygen,carbon
iv)1s^2,2s^2,2p^6,3s^2,3p^5
2bi)
PHYSICAL PROPERTIES
METAL
i) high melting and boiling point
ii) they are good conductor of heat and electricity
NON-MATEL
i)they have lower melting and boiling point
ii)they are poor conductors of heat and electricity.
CHEMICAL PROPERTIES
METAL
i) they form basic and ampheric oxides
ii)the react by electron lose or donation of electron
NON-METAL
i)the form acidic oxides
ii)the react by sharing and accepting electrons
2bii)
i) Al2 O3(Aluminum oxide)
ii) sodium hydride (NaH)
iii)zinc trioxocarbonate(iv)ZnCo3
iv)silicon tetrachloride (SiCl4)
2ci)
i) variable oxidation states
ii) complex ion formation
iii) they possess strong metallic bonding
2cii)
1s^2,2s^2, 2p^6, 3s^2, 3p^6, 3d^10, 4s^2
2ciii)
Zinc is not considered as a typical transition element bacause it
has only one oxidation state of +2, it metallic ion are not coloured
and it is not used as a catalyst.
2d)
Na2Co3 + MgCl2 —->2NaCl + MgCo3
1mole 1mole 2mole 1mole
molar mass of Na2 Co3 = 2 * 23 + 12 * 1 + 16 * 3 = 46+12+48 =
106g
==================================
5ai)
Na2S2O3 ===>2 + 2x + 6 = 0
2x = 6 – 2 = 4
X = 4/2
X = 2
The oxidation number of sulphur is 2
5aii)
Rhombic&monoclinic
5aiii)
– Both are tetravalent
– both are allotrope of carbon
5bi)
CO2&Chloroflorocarbon
5bii)
There is increase in sun radiation reaching the earths surface ie
Global warming
5biii)
ThunderStorm
5iv)
I2KNo3. ——–>2KNO3 + O2
2AgNo3 ———>. 2Ag + 2No
5ci)
Calcium chloride in a solution can give rise to crystal using
filtration and evaporation to dryness. The sol is filtered into
filtrate and residue b4 evaporation to dryness takes place.
5cii)
– Because of presence of hydrogen bonding in NH3
– Because Iodine as higher molecular mass than chlorine
5di)
Mol of Nacl = Mass / MM
= 5.85/ 58.01
= 0.1mol
From the equation
2mol of Nacl gives 2mol of HCL, 0.1mol of Nacl gives 0.1mol of
Hcl
Vol of Hcl = 0.1 x 22.4 = 2.24mol/dm^3
================
Practice Waec Chemistry Questions On Objective
If you can be able to access these questions with the appropriate answers without using textbooks or materials you are 80% ready for the 2023 Chemistry Exam. Kindly submit the answer in the comment section below and if you are interested in the 2023 Waec Chemistry answers kindly show interest.
The components of universal indicator solution can best be separated by
A. Chromatography
B. Filtration
C. Evaporation
D. Crystallization
E. Fractional distillation
The components of universal indicator solution can best be separated by
A. Chromatography
B. Filtration
C. Evaporation
D. Crystallization
E. Fractional distillation
The number of replaceable hydrogen atoms in one acid indicates its
A. Basicity
B. Acidity
C. Alkalinity
D. Reactivity
E. PH value
Catalytic hydrogenation of alkenes produces compounds with the general formula
A. CnH2n+1 OH
B. CnH2n+1
C. CnH2n+2
D. CnH2n-2
E. Cx(H2O)y
A measure of the degree of disorder in a chemical system is known as the
A. Enthalpy
B. Free energy
C. Activation energy
D. Entrophy
E. Equilibrium
Which of the following occurs when an aqueous solution of sodium hydroxide is electrolysed using graphite electrodes?
A. Sodium metal is produced at the anode
B. Sodium amalgam is formed at the cathode
C. Oxygen gas is produced at the anode
D. The grahite anode dissolves
E. The resulting solution becomes acidic
If the volume of a given mass of gas at 0oC is 27.3cm3, what will be the volume of the gas at 10oC, pressure remaining constant?
A. 2.73cm3
B. 28.3cm3
C. 37.3cm3
D. 273cm3
E. 283cm3
Glucose can be obtained from starch by
A. Hydrogenation
B. Dissoiation
C. Hydrolysis
D. Dialysis
E. Dehydration
How many faradays of electricity are required to liberate 9g aluminium? (AI = 27)
A. 0.1
B. 0.3
C. 1.0
D. 2.7
E. 3.0
When water is dropped on calcium carbide, the gaseous product is an
A. Alkane
B. Alkene
C. Alkyne
D. Alkanol
E. Alkanal
Practice Waec Chemistry Questions On Theory
a) Define each of the following terms and indicate one use of each:
(i) Nuclear fission; (ii) Nuclear fusion.
(b) Alpha particle emission by 29325� proceduces an element A. Beta particle emission by the particle A produces another element B. Element B also undergoes alpha particle emission to produce 22789��. Write balanced equations to represent the above statement.
(c) The models below represent the filling of orbitals in an atom.
State which rule(s) is/are violated or obeyed by each model.
(d) Explain why the boiling point of H2S with relative molecular mass of 34 is lower than that of H2O with relative molecular mass of 18.
(e) HCI is passed into each of the following solvents:
(i) water;
(ii) methylbenzene. I. State the effect of each solution on blue litmus paper II. Compare the electrical conductivities of the two solutions.
(f) Zinc dust is added to copper (II) tetraoxosulphate (VI) solution. State;
(i) what is observed; (ii) the type of reaction that occurs.
Question 2 :
(a)(i) State two differences betwecii the properties of solids and gases
(ii) What process does each of X, Y and Z represent in the changes shown below?
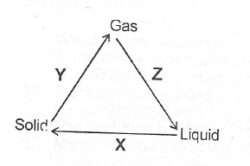
(b)(i) State Charles’ Law (ii) Draw a sketch to graphically illustrate Charles’ Law.
(c) 60 cm of hydrogen diffused through a porous membrane in 10 minutes. The same volume of a gas G diffused through the same membrane in 37.4 minutes. Determine the relative molecular mass of G. [ H = I ]
(d)(i) State two assumptions X of the kinetic theory.
(ii) Consider the reaction represented by the Solid or Liquid following equation:
H2(�) + Cl2(�) → 2HCI(�)
Use the kinetic theory to explain how the rate of formation of HCI(�) would be affected by I. increase in temperature; II. decrease in pressure.
(e) Given different examples, mention one metal in each case that produces hydrogen on reacting with (i) dilute mineral acid; (ii) cold water; (iii) steam; (iv) hot, concentrated alkali.
The 2023 Waec Chemistry Theory and objective questions and answers are loading! 2023 Chemistry Essay answers Loading!! 2023 Waec Chemistry objective Answers Loading!!! Kindly bookmark the website for the answers that will be released. or better still reload the site to check if the answers for the 2023 Waec Chemistry questions and answers have dropped.
Before you leave this page kindly make sure you understand and know how WASSCE grades your WAEC Subject. The reason why we are doing this, is not to frighten or scare you but to put you in check and see reasons why you need to be serious with your Government study for the 2023 Government Exam
Waec Grading System For all Waec Candidates
Do you know that the West African Examination Council (WAEC) Board has published the Waec grading system of results? Kindly check below to see the meaning of the Waec Grading Result.
The table below shows candidates’ positioning of the Waec grading of results ranging from A1, B2, B3, C4, C5, C6, D7, E8, F9 are the complete list of Waec Grading Result. All Waec candidates must fall into one of the Waec Grading Systems.
| GRADE | NUMERIC VALUE | INTERPRETATION |
| A1 | 1 | EXCELLENT |
| B2 | 2 | VERY GOOD |
| B3 | 3 | GOOD |
| C4 | 4 | CREDIT |
| C5 | 5 | CREDIT |
| C6 | 6 | CREDIT |
| D7 | 7 | PASS |
| E8 | 8 | PASS |
| F9 | 9 | FAIL |
WAEC Grading Percentage Scores
- A1 Excellent 75% – 100%
- B2 Very good 70% – 74%
- B3 Good 65% – 69%
- C4 Credit 60% – 64%
- C5 Credit 55% – 59%
- C6 Credit 50% – 54%
- D7 Pass 45% – 49%
- E8 Pass 40% – 45%
- F9 Failure 0% – 44%
If you have been wondering where to get the best and latest Waec news updates and guide about Waec 2023, how to pass Waec, a timetable for 2022/2023 Waec, free online and hardcopy Waec past questions, and hot topics to read for Waec, latest news updates and Waec CBT practice platform, then subscribe to the newsletter or join our Forum
If actually, this information is awesome and useful to you please kindly share using via Facebook, WhatsApp, Twitter, and Google+


38 Comments
Please answer some of these questions for me
If are are interested in Waec Chemistry Practical Answers
WhatsApp us now
09035742503
TO GET THE 2023 WAEC CHEMISTRY QUESTIONS AND ANSWERS,CALL OR MESSAGE 08024485178 ON WHATSAPP NOW…….
NO TIME TO WASTE …..
We are recommending all our users to WAEC Worker who helped people on physics
09035742503
Chat him for help
All that to him
Am interested
Those of you that want to receive chemistry Practical Answers fast
WhatsApp us now not free
09035742503
TO GET THE 2023 WAEC CHEMISTRY QUESTIONS AND ANSWERS,CALL OR MESSAGE 08024485178 ON WHATSAPP NOW…
NO TIME TO WASTE …..
please I need it
Am ready
I need it
Check your gmail
Please, kindly notify me when the correct questions and answers for chemistry WAEC 2023 have dropped
I want
Please help me send chemistry answers for 2023waec
How can i get the question and answers for chemistry
WhatsApp me
You’ll get WAEC 2023 theory Questions and Answers immediately.
WhatsApp 07085160362
TO GET THE 2023 WAEC CHEMISTRY QUESTIONS AND ANSWERS,CALL OR MESSAGE 08024485178 ON WHATSAPP NOW.
NO TIME TO WASTE
2023 WAEC theory Questions and Answers
WhatsApp 07085160362
Get the answers an hour before the exam.
Do you have for chemistry theory too??
Chemistry theory
WhatsApp me on 07085160362
You’ll receive answers 1hr before the Tim for the exams.
I just WhatsApp you sir
TO GET THE 2023 WAEC CHEMISTRY OBJ & THEORY QUESTIONS AND ANSWERS,CALL OR MESSAGE 08024485178 ON WHATSAPP NOW…
NO TIME TO WASTE …..
Is the question real
But I cannot do it because it my mummy phone please help
I am interested in the chemistry essay and objective for 2023…
Ok
I just send you a message
And the question should be send to me before tomorrow so I can do some practice
And the question should be send to me before tomorrow so I can do some
Pls how can i get chemistry Question before the exam
2023 WAEC CHEMISTRY OBJ & THEORY QUESTIONS AND ANSWERS ARE NOW AVAILABLE..
TO GET IT,CALL OR MESSAGE 08024485178 ON WHATSAPP NOW…
NO TIME TO WASTE….
2023 WAEC CHEMISTRY OBJ & THEORY QUESTIONS AND ANSWERS ARE NOW AVAILABLE..
TO GET IT,CALL OR MESSAGE 08024485178 ON WHATSAPP NOW……
NO TIME TO WASTE…….
Please i need in now
Please what if I don’t have WhatsApp account
Like aw many minutes before the exam and is it free
Please if there is waec group or your group add me up 08052263004
what about the ones above are they not correct ?
I enjoyed the site…and want to be participating in this site…..
1A,B,CDCBCDB
yes